What is involved in a Brain Machine Interface (BMI; also called Brain Computer Interface or BCI) set-up? Here is a primer on paradigms for BMI or BCI.
The idea of controlling a computer or a machine with the brain has always caught the fascination of humankind, churning out many science fiction novels and movies on this topic, even as early as in the 1950s. As we already know, controlling an external device or computer using your brain is no longer the stuff of science fiction.
An early demonstration of Brain Machine Interface
One of the early demonstrations of this was in 1999 by Chapin and colleagues, where it was shown that a group of cortical neurons could directly control a robotic manipulator [1]. The figure below shows the experimental setup in [1], where neural signals (g and h) recorded with electrodes implanted in the primary motor (MI) cortex and ventrolateral (VL) thalamus, when the rat presses the lever (b) to obtain water are transformed into a ‘ single signal’ (i) that predicted the forelimb movement, which was then used to manipulate the robot arm (c), allowing the rat to obtain water.
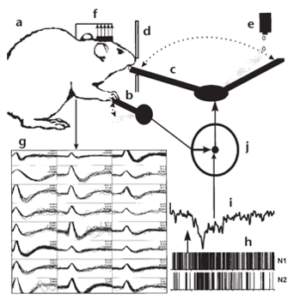
Figure 1 : Experimental setup described in [1]
This simple experiment already hinted at the considerable potential of what is known as brain machine interface (BMI) (also known as brain computer interface (BCI)) to help restore motor functions in severely handicapped / paralyzed patients. This has led to continuous research and development in the field of BMIs and have made BMIs a central component of systems neuroscience, with applications ranging from control of external devices such as wheelchairs, rehabilitation robotics as well as the potential for prediction of onset of seizures in epilepsy or tremor in Parkinson’s disease to healthy users in the area of games.
The Brain Machine Interface set-up
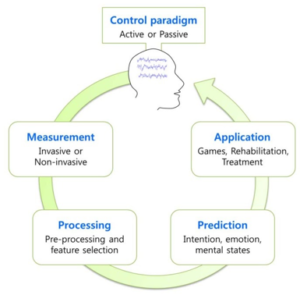
Simply put, a BMI (or BCI) is a device that translates neural activity into commands to control an external machine. As shown in the figure (from [2]), a BMI mainly contains three parts –
1) A device to record neuronal activity – this could be invasive (for example ECoG) or non-invasive (e.g. EEG), which would be generated due to, for example, user imagining movement of a body part (active control) or due to natural interaction of the user with the environment without any any additional effort from the user (passive control)
2) An external system that needs to be controlled. This could be something as simple as a cursor on the computer screen or as complex as a prosthetic limb.
3) An algorithm that can translate multichannel neural signals into a control command and this algorithm acts as sort of a bridge between the neural signals and the
device/system to be controlled. More specifically, after pre-processing the recorded neural signal (for example using EEG ), features are extracted from these signals in order to represent them in a compact form. Several classification algorithms are available to classify these features (such as support vector machines, linear discriminant analysis in addition to advanced machine learning and deep learning methods, which can extract additional features to improve classification), before being translated into a command signal.
Recording techniques for BMIs
What kind of neuroimaging methods can work for BMI applications? From a practical perspective if the goal is to enable movement in those who have paralysis or motor neuron degeneration, fundamentally it has to work in a way that is portable and not constrain movement. It also needs to operate on the millisecond time scales of movement. This rules out non-invasive recording techniques such as magnetoencephalogram (MEG), functional magnetic resonance imaging (fMRI) and functional near-infrared spectroscopy (fNIRS). fMRI and NIRS measure neural activity indirectly i.e., based on the blood oxygenation level and have poor temporal resolution while the bulky size and constraint of movement imposed by MEG or fMRI makes them impractical. This leaves electrophysiological recordings.
see related post 7 Ways to Peer into the Living Human Brain
Invasive implanting of electrode arrays that measure intracortical activity such as single-unit activity, multi-unit activity and LFP have been used with fairly good success in animals (Neuralink for example claims to do this with greater resolution with its neural lace and has been demonstrated in pigs). In humans, electrode arrays such as Micro-ECoG [4] which is more recently developed technology, has better spatial resolution than traditional ECoG arrays [5-6]. Epidural ECoG which is less invasive than ECoGs has also been successful and can reduce the risks associated with highly invasive ECoGs like stroke, hemorrhage as well as provide long-term signal stability.
What about EEG? Can it be used effectively in BMI applications? While EEG offers millisecond temporal resolution, which is often useful in BCI applications, it suffers from poor spatial resolution (in the range of cm) which can pose a hindrance to picking up specific motor signals. However, it has been shown to work reasonably in a range of other kinds of BMI applications which will be discussed in subsequent posts.

