Optogenetics have been used for bi-directional BCI in mice but is unsuitable for freely moving subjects due to its tethered optical fibers or fixed imaging and invasive nature – challenges that might be solved by a hybrid opto-microECoG array.
In the previous blogpost we discussed the advantages of bidirectional BCI using electrical stimulation as well as the challenges they pose. To overcome the challenges posed by electrical stimulation, optical stimulation has been proposed. Here we will mainly focus on optical techniques that use genetically modified neurons (optogenetics) as opposed to techniques that exploit the interrelation between neural activity and hemodynamic response (ex NIRS, laser doppler flowmetry).
Optical techniques provide sub-millisecond temporal resolution along with cell-type specificity. Laser or light emitting diode (LED)-coupled optical fiber or micro-LED arrays are most commonly used optical techniques. Optical stimulation is considered to be more specific than electrical stimulation (see the Figure below) and does not cause any stimulation artifacts, as with electrical stimulation. In addition to stimulation, light can be used to control and measure neuronal activity, to achieve bidirectional BCI [ 1].
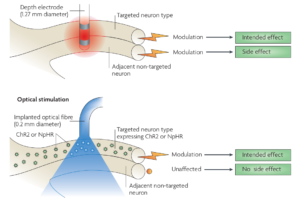
Figure 1 : Optical stimulation is more specific compared to electrical stimulation, which can also activate adjacent neurons. [2]
The basic idea here is to use light to interact with genetically modified neurons, referred to as optogenetics. For instance, green fluorescent protein can be bound to other proteins so as to make it responsive to intracellular calcium in neurons, making them fluoresce when activated. Using imaging sensors, the activity of a population of neurons can then be monitored. For stimulation, a neuron can be genetically modified to express ion channels that are sensitive to light of different wavelengths and either open or close, resulting in excitation or inhibition of a neuron. As shown in illustration below, Channelrhodopsin-2 (ChR2) , a cation channel, opens when exposed to blue light (~470 nm) allowing Na+ ions to enter the cell. On the other hand Natronomonas pharaonis halorhodopsin (NpHR), a chloride pump is activated when exposed to yellow light (~580 nm) allowing Cl- ions to enter the cell. In Figure 2c we can see that when blue light is flashed, neurons are activated, whereas the yellow bar denoted the inactivation due to yellow light exposure.
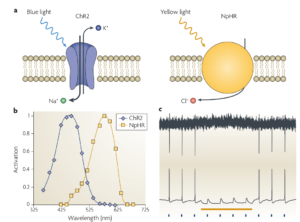
Figure 2 : Illustration of how channels expressed on genetically modified neurons react to light exposure of different wavelengths. [2]
Prsa et al. developed an all-optical bidirectional BCI,with wide-field two-photon population imaging of neurons expressing genetically encoded calcium (Ca) indicators in primary motor cortex (M1) and simultaneous activation of neurons expressing optogenetic actuators in primary somatosensory cortex (S1) of a mice [3]. However, optical techniques are unsuitable for implementing chronic bidirectional BCI in freely moving subjects, mainly due to tethered optical fibers or fixed imaging systems and its size [4] and also their invasive nature.
Hybrid approach
Recently an opto-microECoG array [4] has been proposed as a hybrid approach for bidirectional neural interface, by combining optical and electrical techniques (See Figure 3). The array consists of transparent epidural electrodes (200 micrometer in diameter) and embedded LED for neural stimulation, packed inside parylene-C material (for biocompatibility). In [4], this array was implanted on the visual cortex of a rat and it was shown that optical LED stimulation can stimulate even deeper brain areas (600 micrometer depth). Also the bidirectional interface showed short-term stability and suggested possibility for chronic implantation.
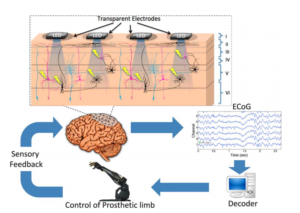
Figure 3 : Hybrid approach combining electrical and optical techniques [4].
One of the major concerns with this setup could be the heat production, since the epidurally implanted LED array is directly interfaced with the brain tissue. In this study, it was observed that as the input voltage and activation duration increased, significant heat was genrated by the LED. For example, with input voltage of 3.2 V for 100 ms, the maximum increase in temperature was around 9 degree celsius. In order to control the temperature rise within safety limits (within 0.5 degree celsius), it was shown that an input voltage of 2.7V with short pulse duration (10-100 ms) was needed. Although no tissue damage was reported, the authors suggest a more systematic investigation with regard to this.
Future avenues
As an alternative to metal electrodes (ECoG) and optical stimulation, Vitale et al. proposed bidirectional neural interface using carbon nanotube fiber electrodes [5]. Carbon nanotubes are particularly interesting because they have 1) high mechanical strength, 2) high electrical conductivity and 3) are biologically compatible.
It has also been demonstrated that microelectrodes made from carbon nanotube fibers display high resistance to biofouling compared to conventional carbon nanotube [6]. In vitro results show that, compared to metal electrodes, the contact impedance is much lower. This makes carbon nanotube fiber electrodes ideal for recording single-neurons. Also, in vivo studies using parkinsonian rats showed that these fiber microelectrodes can effectively stimulate neurons, similar to metal electrodes but with reduced inflammatory response. Also in vivo recordings showed that the signal-to-noise ratio reached stability after about 1 week and there was no signal degradation for 4 weeks, demonstrating the possibility and suitability of chronic recordings as the. All these features make carbon nanotube fiber based microelectrodes an extremely interesting alternative for bidirectional interface that overcomes many challenges associated with present bidirectional systems, including power, size, long-term stability and biocompatibility.
References
[1] Kwon KY, Lee HM, Ghovanloo M, Weber A, Li W (2014) A wireless slanted optrode array with integrated micro leds for optogenetics. In: 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS). IEEE, pp 813–816
[2] Zhang, Feng, et al. “Circuit-breakers: optical technologies for probing neural signals and systems.” Nature Reviews Neuroscience 8.8 (2007): 577-581.
[3] Prsa, Mario, Gregorio L. Galiñanes, and Daniel Huber. “Rapid integration of artificial sensory feedback during operant conditioning of motor cortex neurons.” Neuron 93.4 (2017): 929-939.
[4] Kwon, Ki Yong, et al. “Opto-μECoG array: A hybrid neural interface with transparent μECoG electrode array and integrated LEDs for optogenetics.” IEEE transactions on biomedical circuits and systems 7.5 (2013): 593-600.
[5] Vitale, Flavia, et al. “Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes.” ACS nano 9.4 (2015): 4465-4474.
[6] Harreither, Wolfgang, et al. “Carbon nanotube fiber microelectrodes show a higher resistance to dopamine fouling.” Analytical chemistry 85.15 (2013): 7447-7453.