After decades of being ignored, the important role of glia in shaping network activity and behavior is redefining how we understand the brain.
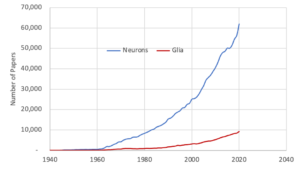
For decades neurons have taken center stage in the study of the brain so much so that the study of the brain has been named for them as ‘Neuro’science. On the other hand, glial cells, although comprising over half the cells in brain, have been largely ignored. In the 1980s and 90s, for every paper published on glia almost 10 were published on neurons. Today the ratio is more like 6.5:1. The consequence is that neurons have largely been studied in isolation and little is known about glia. A decade from now this will probably be seen as one of the major failings of neuroscience.
The growing understanding of Glia (in rodents)
When I was a graduate student two decades ago, the prevailing view was that glia were little more than housekeeping cells and were irrelevant to things like the brain’s network activity, its plasticity, information processing and ultimately any behavioral consequences. One big reason that they were so disregarded was that glia are not excitable cells like neurons – which means they don’t transmit action potentials or electrical spikes that can travel long distances. Action potentials are of course a key part of brain function but it’s debatable if they are the only thing that is important (see related posts Transport of Proteins, RNA and DNA among Brain Cells). Today the picture of glia that is emerging is telling a whole different story [1, 2].
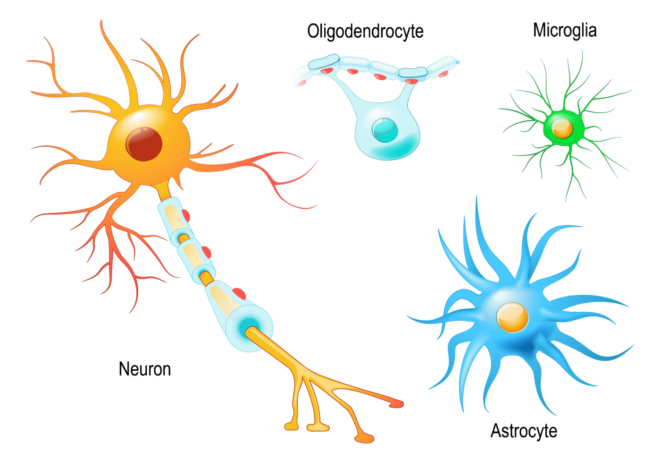
At a cellular level, astrocytes, a type of glial cell, have now been found to do all sorts of things. Astrocytes are always present at any neuronal synapse, playing a key role in neurotransmission. They sense the behavior of neurons through all kinds of channels and receptors and respond with elaborate signaling with calcium waves. They release all sorts of signals that modulate the transmission between neurons, control the resources available to neurons by regulating blood flow and a whole host of other things. Astrocytes have everything to do with how many synapses form, how and which ones are pruned and modulate synaptic strength by regulating receptor composition on both sides of the synapse.
For much of the early 2000s this research focused on the behavior of single astrocytes with the assumption that they were a pretty homogeneous type of cell. Turns out they are enormously heterogeneous in their gene expression and function and the bigger story may be what they do as a network.
For one thing, each astrocyte connects to and regulates a large number of neurons and without astrocytes there is disrupted connectivity and synchronization, at least in the cerebellum [3]. Second, astrocytes connect to each other through a gap junctions that allow the passive transmission of charge. How does this affect network behavior?
Glia and Network Behavior
Since the field of neuroscience has only just woken up to the significance of glia in brain function, not a whole lot is understood. It is also significant that the biggest differences between mouse and human brains is not in their neurons but in their glia – which are much more powerful in humans and have gene expression more similar to mouse neurons.
See related posts From Mouse Brain to Human Brain and Astrocytes in Charge
Nonetheless what we are beginning to understand in mice is starting to hint at glia as central network players, responsible for certain behaviors quite distinct from the simplistic models that pervade popular thinking. For instance, how does the brain’s network incorporate the past into its computations if neurons simply fire immediately in response to a stimulus? One proposal has been that maybe glia play a role in mediating responses on different timescales from seconds to minutes (on their own, neuronal responses are typically on the timescale of milliseconds) [4].
How this could work is still not known but some evidence is starting to emerge. One phenomenon that has been observed that may play into this is something called barrage firing – a kind of persistent or long lasting firing on the order of tens of seconds in the distal axon or far from the cell body, which occurs in inhibitory neurons after a whole lot of repeated stimulation. This is weird of course from the perspective of the classical integrate and fire neuron where the firing initiates at the cell body within milliseconds of a stimulus. So how does it happen? The specifics are not clear but, you guessed it, astrocytes are behind this behavior [5]. Somehow they integrate the neural activity over long times scales to drive action potentials in the distal axons. Altogether this means that networks of astrocytes play a big role in the spatiotemporal dynamics of neural networks.
Then there are microglia. These are the brain’s resident macrophages thought to be mainly involved in immune response that do things like remove dying neurons. These cells too have now been found to control network behavior. Getting rid of them in mice results in all hell breaking loose with network activity becoming amplified and over synchronized, leading to seizures [6].
Incorporating Glia into Models
Much still has to be learned about glial networks and their interaction with neurons. However what is clear is that our models of brain networks will need a substantial overhaul. No computational model will have any real relevance to the behavior of the brain without incorporating glia. Computational neuroscience has been built around modeling networks of neurons as independent integrate and fire units. Going from neural networks to neuro-glial networks will need a complete rethink.
See related Post The Crisis of Computational Neuroscience
References
[1] Pannasch U. and Rouach N. Emerging role for astroglial networks in information processing: from synapse to behavior Trends Neurosci 2013 Jul;36(7):405-17.
[2] Kofuji P. and Araque A. Astrocytes and Behavior Annual Review of Neuroscience Vol. 44:49, July 2021
[3] Kanner S. et al., Astrocytes restore connectivity and synchronization in dysfunctional cerebellar networks Proc Natl Acad Sci U S A . 2018 Jul 31;115(31):8025-8030.
[4] Araque A. et al., Gliotransmitters Travel in Time and Space Neuron. 2014 Feb 19; 81(4): 728–739.
[5] Deemyad T. et al,. Astrocytes integrate and drive action potential firing in inhibitory subnetworks Nat Commun. 2018 Oct 18;9(1):4336.
[6] Badimon A. et al., Negative feedback control of neuronal activity by microglia Nature 2020 Oct;586(7829):417-423.

I am at once encouraged and discouraged to read this article on Google today: encouraged because I have, as a layperson diagnosed with AD (currently at the MCI stage) read enough neuroscience articles to know about glial cells, and discouraged because I see that they have been studied for years but the information has not risen to the top of neuroscience research. I am convinced that these cool little cells are really the key performers!
If possible, could you please keep me updated on your progress, and let me know of any clinical trials involving glial cells, as I would be most eager to participate! (I review clinical trials daily via Alzheimer’s Association’s Trial Match program, but I have not yet seen one involving glial cells.)