Neuronal avalanches are a structure of organization of cascades of synchronized of activity in the cortex that have several surprising implications for how we understand information transmission.
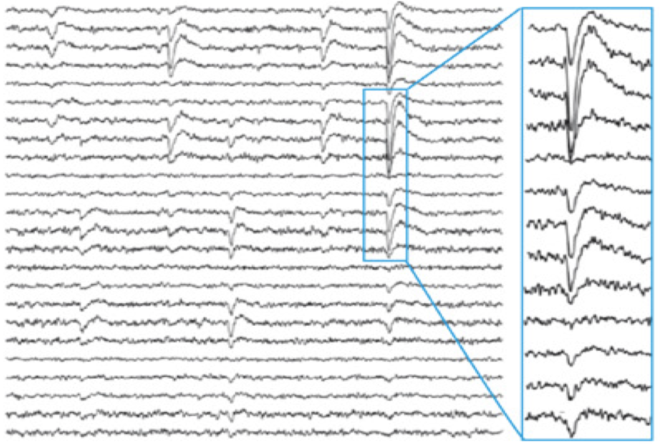
Neuronal avalanches describe the structure of organization of cascades of synchronized of activity in the cortex that has several implications for how we understand cortical activity. It was first characterized by John Beggs and Dietmar Plenz in an organotypic in vitro preparation from mice [1, 2]. In this preparation 300 micron thin cortical slices from newborn mouse pups were plated on 64 channel microelectrode arrays with 30 micron diameter electrodes, where still migrating cells grew out over a two week period to cover the electrodes in a pattern that mimicked intact layer 2/3 cortical architecture but in 2-D form. Remarkably, and entirely unexpectedly, after two weeks, during which time the cultures were placed in a dark incubator with no electrical stimulation, synchronous events began to occur that could be visually identified as sharp negative deflections in the local field potential (LFP) recordings (Panel A below from [1] ).
 see related post Brain in a Dish
see related post Brain in a Dish
Beggs and Plenz noted both the timing and amplitude of these negative LFP deflections (nLFPs) in raster plots (panel B above from [5]) and characterized how they were grouped in time. All simultaneous and consecutive nLFPs were grouped into an event. The end of an event was marked by an empty time bin with no nLFP peak. They then looked at how these events were organized in space and time.
Scale invariant organization of synchrony
Remarkably, the size of these events organized into a statistical structure where the probability of finding an event of size s, P(s), was inversely proportional to its size s.
![]()
This was the case regardless of whether s was considered to be simply the number of nLFPs (the number of sites participating in an event) or the sum of their amplitudes (the total amount of synchrony measured across all sites). This statistical structure is called a power law and is a long tailed distribution that looks like a straight line when plotted on a log-log scale as shown in the Fig. below from [1]. The implication is that there is no length or spatial scale like there is in a normal distribution, for example, where the occurrence of an event of a particular size depends on a characteristic mean size. Thus knowing the exponent alone you can exactly predict how many events of a particular size will occur over a long enough period of time.
Essentially, regardless of how you sliced and diced the measurement by removing every other electrode from the grouping or looking at just one section of the array, this structure still held up (Panel B). Essentially it was invariant to the spatial scale or resolution. What this meant was that the largest size of an event was constrained only by the size of the system [2] which can be seen by the way the distribution sharply drops off at the value represented by the number of electrodes at which activity was measured. This statistical pattern of occurrence of periods of synchrony were termed neuronal avalanches for their similarity to the behavior of ‘avalanches’ of sand in the famous sandpile model demonstrated by Bak, Tang and Weisenfeld [3, 4].

The specific value of the exponent, alpha , however was dependent on the time scale used to resolve the events (See Figure below adapted from [1] and [5]. If the recording was carried out at 1000 samples per seconds, the bin size delta t would be 1 ms. However, as bins were collapsed together to create a coarser and coarser time resolution, or larger values of delta t, the exponent decreased systematically [1, 5]. This statistical characteristic was therefore not just invariant to the spatial scale, it was also invariant to the temporal scale, meaning there is no characteristic time scale in this cortical activity – events can form on all time scales. Essentially it was multifractal in nature and had the profound implication that one could identify this structure regardless of the spatial or temporal scale of measurement.

see related post From Neuron to Brain: The Perils of a Reductionist Approach
Artifact of the Dish or Universal Phenomenon of the Cortex?
This neuronal avalanche structure would never have been discovered were it not for the very obvious negative deflections in the local field potential (LFP) recordings in the dish that stood out to the eye. LFP recordings in vivo look considerably different. The image below from Petermann et al shows LFP recordings from awake monkeys using chronically implanted microelectrode arrays.

Yet despite the different and more complex visual appearance of in vivo LFP recordings, the spatial and temporal structure of negative deflections were preserved in awake monkeys [5] and even in human ECOG recordings [6]. Thus, all evidence points to neuronal avalanches as a universal statistical feature of the mammalian cortex that is conserved across species and spatial scales.
A significant difference across species from rodents to monkey, however, was in the relationship between how fast the exponent changed with the time resolution, [5]. In monkeys the exponent changed much less steeply than in mouse and rats (see panel B in figure directly above) which suggested that large events on longer time scales were more likely in monkeys than rodents.
see related post From Mouse Brain to Human Brain
Active or passive process?
One question that comes up frequently is whether this is simply a feature that arises by the passive spread of electrical activity or volume conduction. While it may seem that way at first several aspects unequivocally demonstrate that it is not. First, volume conduction will have characteristic spatial and temporal scales that are determined by how fast the spread of charge diffuses in space. Second, cascades arising from volu
me conduction would be more spatially compact whereas the avalanche  structure does not change when you measure at electrodes further apart and individual avalanches are often observed on non-contiguous electrodes (i.e. it can ‘hop’). Finally, and perhaps most significantly, this organization can be disrupted by the application of drugs that change the balance of excitation and inhibition [1] (see panel on the left, picrotoxin blocks inhibition). Thus all evidence points to an active process in the cortex that depends on the balance of excitation and inhibition to maintain a scale invariant process of synchronization across the cortex.
structure does not change when you measure at electrodes further apart and individual avalanches are often observed on non-contiguous electrodes (i.e. it can ‘hop’). Finally, and perhaps most significantly, this organization can be disrupted by the application of drugs that change the balance of excitation and inhibition [1] (see panel on the left, picrotoxin blocks inhibition). Thus all evidence points to an active process in the cortex that depends on the balance of excitation and inhibition to maintain a scale invariant process of synchronization across the cortex.
Implications of neuronal avalanches
The various findings relating to the ‘neuronal avalanche’ structure are as follows:
- It occurs in naive mouse organotypic culture and not just in vivo measurements, indicating that it is an intrinsic property of cortical tissue that is not dependent on any external stimulus.
- The power law structure and invariance to spatial resolution indicates that events of all sizes are possible with the largest event size determined not by any characteristic size but rather by the scale of the system – a larger brain can produce larger events.
- The systematic relationship between the power law exponent and the time scale on which avalanches were computed (the bin size or t) slope suggests that these spatially synchronized events can occur on all time scales though larger events were relatively more likely on fast time scales.
- The statistical organization of synchrony as ‘avalanches’ is an active process that depends on the precise balance of excitation and inhibition in the cortex.
- The structure implies a precise branching process or rules governing propagation of activity such that the possible outcomes or patterns in the system are maximal, sometimes referred to as a ‘critical state’ [7-9] where information transmission is optimized [9].
- The difference in the relationship between how fast the exponent changed with the time resolution, in rodents and monkeys suggests that possibly with evolution there has been an increase in the capacity for large spatial synchrony across longer time scales [5]. This may have implications for thinking on longer time scales in the past and future.
So altogether, neuronal avalanches are an intrinsic statistical framework of cortical architecture that is conserved across mammalian species and provides insight into the nature of synchrony and information transmission in the cortex. It is possible that the subtle features of the relationship between the exponent and time scales may be found relate to various aspects of intelligence in future studies.
References
- [1] M. Beggs and D. Plenz, “Neuronal avalanches in neocortical circuits,” J Neurosci, vol. 23, no. 35, pp. 11167-77, Dec 3 2003.
- [2] Plenz and T. C. Thiagarajan, “The organizing principles of neuronal avalanches: cell assemblies in the cortex?,” Trends Neurosci, vol. 30, no. 3, pp. 101-10, Mar 2007.
- [3] Bak, C. Tang, and K. Wiesenfeld, “Self-organized criticality,” Phys Rev A Gen Phys, vol. 38, no. 1, pp. 364-374, Jul 1 1988.
- [4] Bak, C. Tang, and K. Wiesenfeld, “Self-organized criticality: An explanation of the 1/f noise,” Phys Rev Lett, vol. 59, no. 4, pp. 381-384, Jul 27 1987.
- [5] Petermann, T. C. Thiagarajan, M. A. Lebedev, M. A. Nicolelis, D. R. Chialvo, and D. Plenz, “Spontaneous cortical activity in awake monkeys composed of neuronal avalanches,” Proc Natl Acad Sci U S A, vol. 106, no. 37, pp. 15921-6, Sep 15 2009.
- [6] Parameshwaran, N. E. Crone, and T. C. Thiagarajan, “Coherence potentials encode simple human sensorimotor behavior,” PLoS One, vol. 7, no. 2, p. e30514, 2012.
- [7] Massobrio, L. de Arcangelis, V. Pasquale, H. J. Jensen, and D. Plenz, “Criticality as a signature of healthy neural systems,” Front Syst Neurosci, vol. 9, p. 22, 2015.
- [8] L. Shew, H. Yang, T. Petermann, R. Roy, and D. Plenz, “Neuronal avalanches imply maximum dynamic range in cortical networks at criticality,” J Neurosci, vol. 29, no. 49, pp. 15595-600, Dec 9 2009.
- [9] L. Shew, H. Yang, S. Yu, R. Roy, and D. Plenz, “Information capacity and transmission are maximized in balanced cortical networks with neuronal avalanches,” J Neurosci, vol. 31, no. 1, pp. 55-63, Jan 5 2011.

Great entries on all that exciting topics! Thanks a lot
Just a small typo maybe?
“which suggested that large events on longer time scales were more likely in monkeys than rodents.”
isn’t it “less likely”?
Also, the peak in the PDF with added picrotoxin, does it change as one adds more and more electrodes? This peak could be due to saturation, picrotoxin only shifting the cut-off scale to larger events.
Yes, the peak will shift as you add more electrodes. However this is is so in the normal case as well since all events larger than the number of electrodes you are measuring from add together in the last bin. You can see this in the second set of figures above where the number of electrodes are systematically changed. That said, the important aspect in the picrotoxin case is that you lose the scale free structure and instead of a continuous power law distribution with a small edge effect in the last bin you get a deviation of the power law where far more events end up being extra large and pile up in the peak at the end.